Case study Questions on Class 9 Science Chapter 4 are very important to solve for your exam. Class 9 Science Chapter 4 Case Study Questions have been prepared for the latest exam pattern. You can check your knowledge by solving case study-based questions for Class 9 Science Chapter 4 Structure of Atom
In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage-based as well. In that, a paragraph will be given, and then the MCQ questions based on it will be asked.
Structure of Atom Case Study Questions With answers
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 4 Structure of Atom
Case Study/Passage Based Questions
The maximum number of electrons that are permitted to be assigned to an energy shellb of an atom is called the electron capacity of that shell. The distribution of electrons in different orbits or shell is governed by a scheme known as Bohr-Bury scheme. According to this scheme :
(i) The maximum number of electrons that can be present in any shell is given by the formula 2n2 where n is the number of energy levels.
(ii) The maximum number of electrons that can be accommodated in the outermost shell is 8. Electrons are filled in the shells in a stepwise manner in increasing the order of energy of the energy shell.
What is the maximum electrons capacity of the N shell?
(a) 24 (b) 8 (c) 18 (d) 32
Answer: (d) 32
Identify the element with the configuration K-2, L-8, M-3.
(a) Aluminium (b) Magnesium
(c) Sodium (d) Beryllium
Answer: (a) Aluminium
Which of the following configuration represent sodium?
(a) 2, 8, 4 (b) 2, 8, 5
(c) 2, 3 (d) 2, 8, 1
Answer: (d) 2, 8, 1
Case Study/Passage Based Questions
The given diagrams show the atomic structures of elements X and Y.
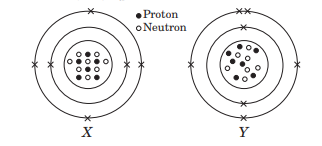
Element X and Y could be _ and _ respectively.
(a) Be and B (b) C and O
(c) F and N (d) C and N
Answer: (d) C and N
Valency of elements X and Y are respectively,
(a) 4 and 3 (b) 2 and 5
(c) 1 and 4 (d) 3 and 4
Answer: (a) 4 and 3
Elements X and Y are
(a) isotopes (b) isoelectronic
(c) isobars (d) isomers.
Answer: (c) isobars
Case Study/Passage Based Questions
The mass of an atom is due to the masses of protons and neutrons in the nucleus. The relative masses of protons and neutrons are almost equal to one. Therefore, the atomic mass of an element should be nearly a whole number. But in many cases the atomic masses are fractional. The main reason for these fractional atomic masses is that these elements occur in nature as a mixture of several isotopes. The atomic mass of an element is the average of the atomic masses of these isotopes in the ratio of their proportion of occurrence.
Chlorine occurs in nature in the form of two isotopes with atomic masses 35 u and 37 u in the ratio of 3 : 1 respectively. The atomic mass of chlorine is
(a) 35.5 u (b) 34.5 u (c) 35 u (d) 36 u
Answer: (a) 35.5 u
An element occurs in two isotopic forms with atomic masses 10 and 11. What is the percentage abundance of two isotopes in the sample having an atomic mass of 10.80?
(a) 20, 80 (b) 50, 50 (c) 25, 70 (d) 60, 40
Answer: (a) 20, 80
The fractional atomic masses of elements are due to the existence of
(a) isotopes having different masses
(b) diagonal relationship
(c) equal number of electrons and protons
(d) none of these.
Answer: (a) isotopes having different masses
Hope the information shed above regarding Case Study and Passage Based Questions for Class 9 Science Chapter 4 Structure of Atom with Answers Pdf free download has been useful to an extent. If you have any other queries of CBSE Class 9 Science Structure of Atom Case Study and Passage Based Questions with Answers, feel free to comment below so that we can revert back to us at the earliest possible
